Sermorelin is a synthetic peptide that stimulates the body to produce more growth hormone, associated with benefits like fat loss, improved body composition, longevity, and more.
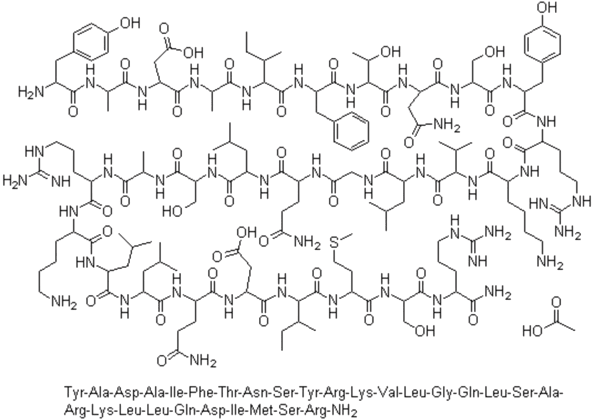
Sermorelin is a synthetic peptide analogue of human growth hormone-releasing hormone (GHRH). It was developed by the German firm EMD Serono for the treatment of growth hormone deficiency (GHD) in both children and adults. The peptide’s primary function is to stimulate the manufacture and release of growth hormone (GH) in the human body.
Following clinical trials, sermorelin was initially approved by the U.S. Food and Drug Administration (FDA) in 1997 and sold under the trade name “Geref,” indicated for the treatment of growth hormone deficiency (GHD) in both children and adults [1, 2]. Its FDA approval was discontinued in 2008 for commercial reasons unrelated to its safety or efficacy [3].
Sermorelin was also used as a diagnostic agent for assessing whether a given patient’s pituitary gland was functioning properly and releasing adequate amounts of GH [1, 2].
The peptide is now being researched for applications beyond the diagnosis and treatment of hormone deficiency, including for its role in sleep quality, heart health, and even as a novel brain tumor treatment.

Sermorelin contains the first 29 amino acids of growth hormone-releasing hormone and is the shortest synthetic peptide to offer the full biological activity of endogenous GHRH [4].
As an analog of GHRH, sermorelin also binds to the growth hormone-releasing hormone receptor found in the anterior pituitary gland, signaling to the pituitary gland to produce and release more growth hormone in the body, by way of somatotropic cells.
Sermorelin’s mechanism is different from that of biosynthetic hGH, since injection of the latter can raise GH in the body to supraphysiological levels. On the other hand, the primary function of sermorelin is to mimic native GRF and stimulate GH secretion to the maximum natural level. [4].
Our team conducted a literature review using PubMed, Google Scholar, and EMBASE to identify the relevant benefits and uses of sermorelin. Here are three of the most notable:
Effective treatment of adult-onset growth hormone insufficiency: Sermorelin is an effective and attractive treatment of adult-onset GH insufficiency. Unlike rhGH, sermorelin does not stimulate IGF-1 production in the liver and instead stimulates the pituitary gland to produce and secrete endogenous hGH, giving it a number of clinical advantages over exogenous hGH.
Notably, according to a 2006 article by Richard Walker, overdoses of endogenous hGH are “difficult if not impossible to achieve” as the effects of sermorelin are regulated by negative feedback involving the inhibitory neurohormone, somatostatin.
Sermorelin is not associated with the pro-cancer and pro-diabetes side effects linked to long-term hGH therapy, and is thus heavily researched to treat adult-onset GH insufficiency along with being used in longevity medicine for preserving youthful anatomy and physiology [5].
Positive effects on body composition: Sermorelin has been identified as one of several growth hormone secretagogues (GHS) that can help manage body composition in hypogonadal males. In a 2020 paper, Deepankar et al. noted that sermorelin can “significantly improve body composition” (in hypogonadal males) while “ameliorating specific hypogonadal symptoms” such as fat gain and muscular atrophy [6].
While the authors suggest that sermorelin could be used to achieve lean mass gain in both hypogonadal males and men with subclinical hypogonadism, to date, there is no published research that has investigated sermorelin’s effect on body composition in healthy subjects, highlighting an area for further research.
Potential anti-aging benefits: A 1992 study identified sermorelin’s potential anti-aging benefits after nine young male test subjects (22 to 33 years old) and ten elderly male test subjects (60 to 78 years old) were administered the peptide. The study found that short-term subcutaneous sermorelin injections enabled healthy male test subjects to reverse age-related decreases in GH and levels of IGF-I [7].
A 1997 single-blind randomized placebo-controlled trial found that elderly male and female test subjects who received nightly subcutaneous injections of sermorelin for 16 weeks experienced a “significant increase in skin thickness.” The peptide was found to produce significant increases in lean body mass in male test subjects and notable increases in libido and wellbeing. Male test subjects gained an average of 1.26 kg of lean body mass by the end of the trial [8]
Sermorelin and heart health: Sermorelin has been investigated alongside other GHRH agonists as a potential treatment for myocardial infarction scarring in swine [9]. The 2015 study found that sermorelin offered a number of benefits in post-heart attack animal models, which included:
As a result, there is research interest in sermorelin’s heart health-related benefits, namely in its potential to treat problems resulting from cardiac remodeling and ability to heal damage in myocytes following a heart attack.
According to a review of sermorelin’s use in the diagnosis and treatment of children with GHD, once daily subcutaneous doses of the peptide were well tolerated [4]. The review noted that the most commonly reported adverse events were transient facial flushing and injection site pain.
According to Drugs.com, the most common sermorelin side effects are [10]:
Sermorelin currently lacks FDA approval as its approval was withdrawn in 2008 for reasons other than safety and efficacy [3]. Data on the safety of sermorelin for non-medical uses such as improving body composition in healthy subjects remains lacking.
As noted by Prakash and Goa, while sermorelin is a “well-tolerated” GHRH analogue when used as a provocative test of GHD in children, such tests would involve a single intravenous 1 μg/kg body weight dose and tell us very little about the peptide’s long-term safety [4].
According to Walker, sermorelin is potentially safer than exogenous rhGH because it does not stimulate IGF-1 and is virtually incapable of causing overdose, given that the peptide merely stimulates endogenous hGH and its effects are regulated by somatostatin [5].
Research suggests that sermorelin is commonly administered at doses starting from 100-200 mcg per day for objectives like improved body composition, often in combination with other growth hormone secretagogues such as ipamorelin.
When used in longevity research, sermorelin is typically cycled for three to six months.
In terms of timing, Prakash and Goa’s research indicates that in the diagnosis and treatment of GH deficiency in children, it was common to administer sermorelin at night before bed, at least two hours after eating, or first thing in the morning when endogenous GH levels are typically at their peak.
Researchers looking to source high-quality sermorelin online should turn to our top recommended vendor:
Research Peptide
is a US-based vendor that offers research-grade sermorelin in 5mg vials for $40 per unit.
We fully endorse this vendor for the following reasons:
To obtain high-quality sermorelin and other peptides, head directly to Research Peptide.
Sermorelin typically comes in the form of powder and must be reconstituted using bacteriostatic or sterile water.
After being reconstituted, sermorelin is administered subcutaneously.
Follow all standard safety precautions when reconstituting sermorelin, including wiping the tops of vials with alcohol and avoiding contact with the syringe used to pull the bacteriostatic or sterile water. To properly reconstitute the peptide solution, drip the reconstituting liquid down the side of the peptide vial and let the solution dissolve on its own.
Qualified researchers may buy sermorelin online as a reference material for research purposes. In the domain of competitive sport, it is important to note that sermorelin and other growth hormone-releasing hormone analogs are banned by the World Anti-Doping Agency.
Non-qualified researcher or laboratory professionals are generally advised to refrain from purchasing and handling sermorelin.
Sermorelin is no longer FDA-approved, and researchers should note the lack of long-term clinical studies involving sermorelin as a risk of administration. However, available safety data has indicated that this peptide is generally well-tolerated with few to no long-term side effects in individuals with no pre-existing risk factors.
Yes, it is. Sermorelin was approved for the treatment and diagnosis of GHD deficiency in subjects of all ages by the U.S. Food and Drug Administration (FDA) in 1997, and its approval was discontinued in 2008 for reasons unrelated to safety and effectiveness.
Research indicates that sermorelin peptide is an effective growth hormone secretagogue that significantly increases levels of hGH in the body in both children and adults. Sermorelin has also shown promise in other clinical contexts, including muscle wasting, lipodystrophy in HIV-infected patients, and impaired cognition in elderly subjects. It has also shown potential as a treatment for patients with recurrent glioma.
No, sermorelin is not an anabolic-androgenic steroid. It is a peptide analog of growth hormone-releasing hormone.
No, sermorelin does not act to boost testosterone production.
Yes, as a growth hormone-releasing hormone analog, sermorelin is associated with the same effects as hGH therapy, including increased muscle mass.
No, in published studies to date, sermorelin has not been found to cause weight gain. In fact, a primary benefit of sermorelin is its ability to reduce adipose tissue, thereby producing weight loss.
Sermorelin has an array of both proven and potential benefits and uses, and may be used to improve body composition, achieve anti-aging benefits, and promote heart health.
The peptide has been approved by the FDA, and is backed by extensive literature that establishes it as an attractive alternative to hGH therapy.
To purchase sermorelin online, we recommend the vendor with the purest peptides and most secure buying experience online.
Disclaimer: Peptideinfo.net contains information about products that are intended for laboratory and research use only, unless otherwise explicitly stated. This information, including any referenced scientific or clinical research, is made available for educational purposes only. Peptideinfo.net makes every effort to ensure that any information it shares complies with national and international standards for clinical trial information and is committed to the timely disclosure of the design and results of all interventional clinical studies for innovative treatments publicly available or that may be made available. However, research is not considered conclusive. Peptideinfo.net makes no claims that any products referenced can cure, treat or prevent any conditions, including any conditions referenced on its website or in print materials.
To the extent that Peptideinfo.net references a product that is also a prescription medication, Peptideinfo.net does not does not offer medical diagnosis or treatment advice. The contents of Peptideinfo.net are intended exclusively for qualified researchers. Any individual seeking any advice on any prescription medication, or any disease or condition, is advised to refrain from using this site and consult their healthcare provider. Statements regarding products presented on Peptideinfo.net are the opinions of the individuals making them and are not necessarily the same as those of Peptideinfo.net.
Your access to Peptideinfo.net is subject to our terms of use.