TB-500 is a research peptide being studied for its wound healing and regenerative effects.
TB-500 is the synthetic version of a natural compound called thymosin-beta-4. It is a research chemical that has been used in both test-tube and animal studies, along with a small number of human trials, having been found to promote wound healing, muscle repair, joint and tissue strength, and endothelial health.
Thymosin-beta 4 was first identified in 1981 after being isolated from a bovine thymus gland sample [1]. In the early-2010’s, the synthetic version TB-500, which had been developed for veterinary purposes, was rumored to be rampantly used in competitive horse racing — giving horses receiving it a significant competitive edge against other horses. It was at this time that testing measures to detect TB-500 in race horses began to be developed in earnest [2].
TB-500, Thymosin beta-4, and all other derivatives thereof are now banned from competitive horse racing, as well as all sporting competitions subject to the Code of the World Anti-Doping Agency (WADA) [3].
However, research on TB-500 is certainly ongoing, and the peptide is of particular interest for researchers studying anti-aging, wound healing, inflammation, cardiovascular health, among other diverse applications.
Our research guide to TB-500 can be found below, including its potential benefits, known side effects, and other pertinent information about the research chemical.

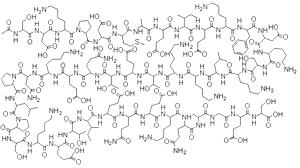
TB-500 is a synthetic version of thymosin-beta 4, a peptide (short chain of amino acids) that has been found in most cells in animals and humans. It is a chain of 42 amino acids (a “tritetraconpeptide”) in the following structure:
Ac-Ser-Asp-Lys-Pro-Asp-Met-Ala-Glu-Ile-Glu-Lys-Phe-Asp-Lys-Ser-Lys-Leu-Lys-Lys-Thr-Glu-Thr-Gln-Glu-Lys-Asn-Pro-Leu-Pro-Ser-Lys-Glu-Thr-Ile-Glu-Gln-Glu-Lys-Gln-Ala-Gly-Glu-Ser-OH.
It is also known as timbetasin, and has a chemical structure of C212H350N56O78S and a low molecular weight [4].
The primary function of thymosin beta-4 is in cell repair and regeneration. It exhibits most of its actions through the sequestering of actin, which — in the simplest of terms — means that it keeps actin in place. In the event of an injury, thymosin beta-4 would sequester actin at the site of the injury, signaling a cascade of other reactions to occur.
Because actin is a key component of connective tissue, this actin-sequestering action of thymosin-beta 4 helps promote connective tissue healing and regeneration. This activity also signals for the body to begin migrating other stem or progenitor cells to the site where repair is needed. Thymosin beta 4 helps those cells to differentiate into, for example, myocytes (muscle cells), epithelial cells, or other connective or vascular cells to repair the area and rebuild the tissue [1, 5].
Much of the existing research on TB-500 and thymosin-beta 4 has been done in test-tube or animal studies. However, the results of these studies point to some potentially promising applications for muscle and connective tissue growth, as well as vascular function.
Note that most of this research refers to the use of thymosin-beta 4, the organic and naturally occurring version of lab-made TB-500. However, TB-500 is structurally and chemically identical to thymosin-beta 4 and theoretically should have the same effects in research subjects.
In some studies, TB-500 may be referred to as synthetic thymosin-beta 4.
Here is a list of potential benefits:
Muscle. One research area with great potential is the effects of TB-500 on muscle, but not just skeletal muscle. Thymosin-beta 4 appears to exert promising positive effects on both skeletal muscle and cardiac (heart) muscle.
In one 6-month mouse study, researchers found that thymosin-beta 4 administration improved skeletal muscle fiber regeneration in mice who were deficient in dystrophin, a connective tissue protein [6].
It also may help to protect cardiac muscle and promote repair after damage to the heart through a number of mechanisms [7].
Connective tissue. Because of its effects on body proteins, TB-500 may also provide some potential benefits for connective tissue like joints, tendons, and the collagen matrices that anchor the skin, muscles, and organs.
In one animal study, researchers treated incision wounds on rats with thymosin-beta 4. They also kept an untreated control group to compare how the wounds healed differently. In the wounds treated with thymosin-beta 4, the researchers noted that there was minimal scarring present compared to the controls, and that the treated wounds were also much narrower in width.
Upon closer inspection, they were able to determine that this was because the thymosin beta-4 treated wounds had more tightly organized collagen fibers that were more mature, while the controls had more disorganized arrangements of collagen fibers that appeared to be less mature.
Thymosin-beta 4 administration appeared to significantly improve collagen structure and hasten its maturity, resulting in improved wound healing and reduced scarring [8].
Vasculature and endothelial function. Vasculature refers to blood vessels, and the endothelium is the inner lining of each vessel that helps to regulate blood flow and blood pressure through constriction and dilation. Healthy blood vessels are flexible with highly functioning endothelial linings.
Animal and test-tube models have indicated that thymosin beta-4 can help promote the growth and development of new blood vessels in a process known as neovascularization, but they can also help promote endothelial cell differentiation to help maintain or restore endothelial function in damaged vessels [9, 10].
Other effects. Other animal studies point to even more potential applications for TB-500 and thymosin-beta 4. One mice study noted that thymosin-beta 4 administration helped to improve blood glucose control and increase insulin sensitivity in mice. Researchers also noted improvements in triglyceride levels with thymosin-beta 4 administration in this study [11].
Thymosin beta-4 has also been used to promote hair growth in mice, so it also shows promise as a follicle stimulator for hair loss conditions [12].
It is important to recall that all of this research is considered pre-clinical, and there hasn’t yet been a great deal of research in humans. Although test-tube and animal studies can reveal some of the potential effects of TB-500, it is important to note that these effects have not been confirmed in human subjects.
Human trials. A handful of high-quality, randomized controlled trials have investigated the effects of thymosin beta-4 administration in humans.
One studied venous ulcers, which are a result of poor circulation and typically manifest on the lower leg. With 73 participants in all, researchers found that a dose of 0.03% synthetic thymosin-beta 4 increased the rate of wound healing, and helped 25% of patients achieve full healing within 3 months [13].
Another investigated the effects of thymosin-beta 4 eye drops for severe dry eye. Among nine participants, researchers found that — after 56 days of treatment — the treatment group had a 35% reduction in eye discomfort, 59% improvement in dry eye testing parameters and increased tear production [14].
There has also been one trial in healthy adults to help assess the safety and potential side effects of thymosin-beta 4, and some early pilot and exploratory studies in regards to thymosin-beta 4 and cardiac function [15, 16].
On the whole, the research to date indicates that TB-500 exhibits minimal to no side effects when administered to research subjects at prudent doses.
The results of one randomized controlled trial in 40 healthy adults – with the express purpose of assessing potential safety concerns with synthetic thymosin-beta 4 – were published in 2010. The researchers found that, in healthy adult subjects, intravenously-administered doses ranging from 42 to 1,260 mg of Tbeta4 appear to be well-tolerated and present minimal risk for toxicity [17]. (Note that the dosages for TB-500 would have been significantly smaller.)
Although there were some adverse events in the course of the study, they were uncommon occurrences and were only mild or moderate in nature. It’s important to note that this was a carefully designed study using only healthy subjects.
Regardless of these preliminary findings, TB-500 should be administered with the utmost caution — by qualified researchers only. Under no circumstances should it be self-administered for experimental or recreational purposes.
The peptide TB-500 has not been evaluated for safety by the United States Food and Drug Administration (FDA), nor by any of its international regulatory counterparts. Accordingly, any statements or findings – formal or otherwise – that TB-500 is “safe” must be taken with a grain of salt.
Nonetheless, in published research to date, TB-500 administration has produced minimal side effects in animal and human studies alike [8, 14, 15, 17].
Many peptides can product injection site pain, lightheadedness, nausea, or even flu-like symptoms in subjects. But there have been very limited reports of TB-500 causing even such minor side effects. On the contrary, it appears that TB-500 is very well tolerated among a range of subjects.
As with any research involving peptides, however, due caution is required when administering TB-500 to test subjects.
Given the relative paucity of published research to date on TB-500, there are no set dosage recommendations for research purposes.
Nonetheless, in the scientific and clinical studies to date, the most common reported dosing range of TB-500 has been 2-5 mg, administered twice weekly, for a duration of 4 to 8 weeks, depending on the nature of the research. Some clinicians favor a higher starting dose for the initial 1 to 2 weeks, followed by a maintenance dose equal to one half of the original dose for the 2 to 6 weeks thereafter.
Alternatively, one human study used a thymosin-beta 4 dose of 0.03% in a gel for the treatment of venous ulcer wounds with favorable results [13].
In research applications, it’s important to use the lowest effective dose, so it’s advisable to start with the lowest dose possible.

For researchers looking to study TB-500, the peptide experts at Peptideinfo.net recommend the following top-rated vendor:
We are fans of Research Peptide due to their strong commitment to quality using independent laboratory testing, as well as their excellent customer reviews, flexible shipping options, and simple reship policy.
Here’s a little bit more about why we like Research Peptide:
On top of that, the research peptides vendor offers capsules and nasal spray formulations. Like this:
Additionally…
Qualified researchers can get 10% off their next order at Research Peptide using our exclusive discount code. Click the button below and enter this code at checkout:
A lab need to be equipped with the necessities for safe and effective peptide research.
To comply with expert guidelines of TB-500 handling, researchers need to have the right supplies at disposal.
These include sterile vials, bac water, and more.
Getting a hold of all the proper ancillary supplies can be challenging for even the most experienced researchers, as few trusted retailers carry what is required.
But it is a necessary step for all researchers.
TB-500 is typically available in the form of powder that should be reconstituted using bacteriostatic or sterile water.
TB-500 is typically administered via subcutaneous injection.
TB-500 should be reconstituted with bacteriostatic or sterile water. It is important to follow all safety precautions during the reconstitution process, including wiping the tops of vials with alcohol wipes and avoiding contact with the syringe used to pull the bacteriostatic or sterile water. To properly reconstitute the peptide solution, take care to drip the reconstituting liquid down the side of the peptide vial. Let the solution dissolve on its own.
TB-500 is a research chemical. At this time, it’s legal to possess only for research purposes and should only be possessed by professionals in the context of in vitro (test tube) testing.
The World Anti-Doping Agency (WADA) considers TB-500 a class S2 “growth factor and growth factor modulator” that is prohibited at all times (in and out of competition, training, off-season, etc.) in the context of competitive athletics.
To gain this designation from WADA, a substance must be currently unapproved for use by governmental regulatory authorities for any use in humans.
Because all of the research on synthetic thymosin beta-4 is pre-clinical at this time, TB-500 falls into this classification.
Although no one can legally possess TB-500 for personal use, competitive athletes of all levels and across all sports must strictly avoid TB-500.
Additionally, although TB-500 has a history of use in competitive horse racing as well, it is also now banned from use in race horses.
TB-500 is a research peptide and should be administered with extreme care. While the peptide has exhibited minimal side effects in published research to date, researchers should note the lack of clinical studies involving TB-500 as a risk of administration.
TB-500 has been shown to effectively stimulate blood vessel growth, promote wound healing, and mitigate oxidative damage in test subjects. Researchers are studying a number of TB-500 applications, including tissue repair, inflammation reduction, injury recovery, and anti-aging.
No, TB-500 is not an anabolic-androgenic steroid; it is a peptide.
No, TB-500 does not act to boost testosterone production.
No, in published studies to date, TB-500 has not been found to build muscle. However, TB-500 has been found to accelerate wound healing, including muscle injuries.
No, in published studies to date, TB-500 has not been found to cause weight gain.
TB-500 is the synthetic version of thymosin-beta 4, a natural healing compound found in human and animal cells.
Its main function is to sequester actin, which sets off a cascade of other reactions in the body aimed at healing, repair, and regeneration of damaged cells, tissues and blood vessels.
Its potential benefits include muscle repair, strengthening of connective tissue, wound healing and improving endothelial function. It may also help with hair loss, dry eye, and insulin resistance. However, research is still in early phases, with little human studies available.
TB-500 should only be used by professionals for research purposes, and the substance (along with exogenous natural thymosin beta-4) has been banned by WADA at all times for use in competitive athletics. It is also banned from competitive horse racing, as it has also seemed to give race horses who received it an unfair advantage.
Overall…
TB-500 shows a great deal of potential in the realms of tissue repair, heart health, and wound healing. We are looking forward to seeing the results of future trials on TB-500.
We recommend buying TB-500 from a reputable source like Research Peptide due in part to their fair pricing, rigorous quality control processes, and solid reputation.
Disclaimer: Peptideinfo.net contains information about products that are intended for laboratory and research use only, unless otherwise explicitly stated. This information, including any referenced scientific or clinical research, is made available for educational purposes only. Peptideinfo.net makes every effort to ensure that any information it shares complies with national and international standards for clinical trial information and is committed to the timely disclosure of the design and results of all interventional clinical studies for innovative treatments publicly available or that may be made available. However, research is not considered conclusive. Peptideinfo.net makes no claims that any products referenced can cure, treat or prevent any conditions, including any conditions referenced on its website or in print materials.
To the extent that Peptideinfo.net references a product that is also a prescription medication, Peptideinfo.net does not does not offer medical diagnosis or treatment advice. The contents of Peptideinfo.net are intended exclusively for qualified researchers. Any individual seeking any advice on any prescription medication, or any disease or condition, is advised to refrain from using this site and consult their healthcare provider. Statements regarding products presented on Peptideinfo.net are the opinions of the individuals making them and are not necessarily the same as those of Peptideinfo.net.
Your access to Peptideinfo.net is subject to our terms of use.