Retatrutide is a novel peptide under investigation for its potential in promoting weight loss, enhancing fat metabolism, and regulating blood glucose levels.
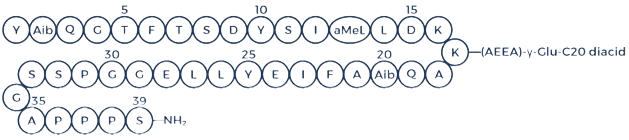
Retatrutide, a synthetic peptide created by Eli Lilly, is also known by its development code LY3437943. This peptide acts as a triple receptor agonist, targeting the glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon (GCG) receptors [1].
Both GLP-1 and GIP are incretin hormones that stimulate insulin secretion and modulate immune responses. Moreover, the activation of GCG receptors by retatrutide is thought to boost metabolic rate and increase energy expenditure [2].
This cutting-edge compound is being explored for its role in controlling blood glucose levels and aiding in weight loss [3].
Important details for researchers:
Although it has not yet been approved by regulatory authorities such as the U.S. Food and Drug Administration (FDA), retatrutide is available to qualified researchers as a reference material for studying the therapeutic potential of this triple receptor agonist.

Retatrutide operates through a unique mechanism as a triple agonist, targeting the GIP, GLP-1, and GCG receptors, with a strong emphasis on GIP receptor activation [4].
Incretin hormones GLP-1 and GIP stimulate insulin secretion from pancreatic beta cells and contribute to feelings of fullness after eating, while glucagon serves a counter-regulatory function by raising blood glucose levels during fasting.
This receptor interaction leads to a comprehensive approach to metabolic regulation, significantly impacting both glycemic control and weight loss. Below is an overview of the underlying mechanisms:
These combined mechanisms make retatrutide a promising option for treating obesity and type 2 diabetes by not only curbing calorie intake but also increasing energy expenditure, offering a dual strategy for effective weight management.
Outlined below you can find several key clinical trials that have demonstrated the efficacy and potential research applications of retatrutide.
As of 2024, phase 3 trials within the TRIUMPH program are in progress, exploring retatrutide’s potential for weight loss. Earlier phase 1 and phase 2 studies have shown promising results, indicating that the peptide could lead to up to a 24% reduction in body weight from baseline.
The initial human trial of retatrutide, a proof-of-concept study, took place in Singapore in 2019. This phase 1 trial involved 47 participants who were administered six different dose levels, ranging from 0.1 mg to 6 mg weekly. The study suggested potential positive effects on appetite, food intake, and other metabolic markers, alongside a favorable safety profile [4].
Following this, a larger phase 2 trial was conducted, lasting 48 weeks and involving 338 non-diabetic adults with overweight and obesity. Participants received varying doses of retatrutide, and the trial demonstrated a dose-dependent reduction in weight and improvement in cardiometabolic risk factors. Key findings included [7]:
All participants who received either 8 mg or 12 mg of retatrutide weekly experienced at least a 5% reduction in their initial body weight. In addition, significant reductions were observed in waist circumference, blood pressure, glycated hemoglobin, fasting glucose, insulin levels, and lipid levels, excluding HDL cholesterol.
Eli Lilly is conducting ongoing research on retatrutide’s potential to manage glycemic control and obesity in individuals with type 2 diabetes (T2D) as part of the phase 3 TRIUMPH clinical trials.
Earlier trials, including phase 1b and phase 2 studies, have shown encouraging outcomes. In a 12-week phase 1b trial, 72 adults aged 20-70 with T2D and baseline A1c levels between 7.0% and 10.5% were treated with either retatrutide, dulaglutide (a GLP-1 agonist approved by the FDA), or placebo.
By week 12, those receiving the highest dose of retatrutide (12 mg weekly) saw a significant decrease in A1c of up to 1.6% and a weight reduction of up to 19.7 pounds (8.96 kg). These results were markedly better than those seen with both dulaglutide and placebo [12].
A subsequent 36-week phase 2 trial involved 281 T2D patients receiving up to 12 mg of retatrutide weekly. Key results include [6]:
Data from phase 2 trials indicate that retatrutide’s side effects are similar in severity and frequency to those associated with other GLP-1/GIP peptide analogs [6, 7].
In one of the phase 2 studies involving 338 non-diabetic participants, gastrointestinal disturbances were the most frequently reported side effects [7].
Here are the most common adverse events experienced by the 62 participants who received the maximum dose of 12 mg of retatrutide weekly:
Serious adverse events were similarly low between the retatrutide and placebo groups, with both at 4%. Among those taking retatrutide, 1% experienced temporary increases in alanine aminotransferase (ALT) levels exceeding three times the normal upper limit.
Additionally, increases in amylase and lipase levels were noted, though these were mostly asymptomatic except for one case of acute pancreatitis in the highest dose group.
Gastrointestinal issues were the leading cause of treatment discontinuation. In the treatment group, dropout rates due to adverse effects ranged from 6% to 16%, depending on the dose of retatrutide administered. There were no treatment-related dropouts in the placebo group [7].
Retatrutide has not yet been approved for any form of human use; however, it is available to qualified researchers for studying its therapeutic potential as a triple receptor agonist.
Retatrutide is currently undergoing clinical development within Eli Lilly’s TRIUMPH phase-3 clinical program, which aims to gather extensive data on its efficacy and safety. This program is designed to provide comprehensive insights that could eventually support regulatory approval for medical use.
However, data from phase 1 and phase 2 trials suggest that the peptide is generally well-tolerated, with side effects similar to those observed with FDA-approved incretin mimetics such as dulaglutide, indicating a comparable safety profile.
Nevertheless, researchers using retatrutide in laboratory settings must remain mindful of potential risks, ensuring that all handling and experimentation are conducted under strict professional supervision.
Retatrutide is not currently available as a prescription medication and has not been approved for human use in the United States or internationally. As of this writing, it remains under investigation in clinical trials for potential applications in treating conditions like type 2 diabetes and obesity, similar to other incretin mimetics.
Qualified researchers and laboratory professionals can legally obtain retatrutide as a reference material for research purposes. However, it is important to note that retatrutide is not available over-the-counter and cannot be used as a dietary supplement.
Unqualified use of retatrutide could lead to legal repercussions, and improper administration carries significant health risks. Researchers should avoid purchasing retatrutide marketed as a supplement or included as an ingredient in supplements, as such products are often of low quality and may pose serious dangers to research subjects.
Researchers are advised to follow specific dosing protocols to ensure the safety and efficacy of retatrutide in weight loss studies. The dosing regimen starts with a low dose of 1 mg per week, which is gradually increased to minimize side effects. The recommended schedule is as follows:
It’s essential that researchers do not exceed the maximum recommended dosage of 12 mg per week. Retatrutide is designed for once-weekly subcutaneous administration, and due to its extended half-life, it can be injected at any time of day, with or without food [6, 7].
In clinical trials, the preferred injection site has been the abdomen, specifically at least 2 inches (5 cm) away from the belly button, to minimize the risk of bruising, redness, infection, or irritation. Studies have shown that retatrutide can be safely administered over a 48-week period, with a safety profile comparable to that of a placebo and other incretin mimetics [6, 7].
This long-term dosing approach indicates that retatrutide does not require cycling on and off, and sustained use is crucial for maximizing its therapeutic effects in weight management and metabolic health improvement.
Researchers looking to buy Retatrutide have several options available from various online vendors. However, it’s important to note that not all suppliers can be relied upon for quality.
For the best results, it’s crucial to obtain Retatrutide from thoroughly vetted sources. Our peptide review team has rigorously tested several suppliers, and the following consistently excel in peptide purity, timely delivery, and customer service:
Polaris Peptides is an emerging supplier specializing in high-quality peptides intended solely for research and development by professionals. This vendor offers several key advantages:
Retatrutide is supplied as a lyophilized powder intended for research purposes and must be reconstituted with either bacteriostatic water or sterile water.
Bacteriostatic water, which contains 0.9% benzyl alcohol, is preferred for reconstitution because it inhibits bacterial growth, allowing the peptide to remain stable for up to four weeks when refrigerated properly at 36 to 46 degrees F (2 to 8 degrees C) [13].
In contrast, when sterile water is used, the peptide’s stability lasts only 24 hours.
Researchers typically follow these steps for the reconstitution process:
Following this protocol ensures proper reconstitution of retatrutide for research while maintaining accuracy and professionalism.
Tirzepatide is a dual receptor agonist that activates both GLP-1 and GIP receptors. It has received FDA approval for the treatment of type 2 diabetes and is under regulatory review for obesity treatment [14].
Although there are no head-to-head clinical trials comparing tirzepatide and retatrutide, early research indicates that retatrutide might lead to greater weight reduction and enhanced glucose control.
This potential advantage is believed to stem from retatrutide’s ability to boost energy expenditure while simultaneously reducing calorie consumption [15].
Retatrutide is not a steroid; it is a peptide composed of amino acids and does not interact with androgen receptors.
Instead, this peptide functions by stimulating GIP, GLP-1, and GCG receptors in various tissues, including the brain, pancreas, gut, and fat cells, contributing to appetite suppression, weight loss, and other metabolic improvements.
Retatrutide and Semaglutide are both peptide-based therapies that target metabolic pathways for weight loss and glucose control. Semaglutide acts as a GLP-1 receptor agonist, enhancing insulin secretion and reducing appetite.
Retatrutide, on the other hand, is a triple receptor agonist that stimulates GLP-1, GIP, and GCG receptors, potentially offering more significant weight loss and improved metabolic outcomes due to its broader mechanism of action. While more research is needed, early studies suggest that Retatrutide might provide superior results in these areas.
Although still in the development stage, retatrutide shows significant potential for various research applications. Currently undergoing phase 3 clinical trials, it is being evaluated for its effectiveness in managing type 2 diabetes and promoting long-term weight control.
Early studies suggest that retatrutide may offer benefits such as improved blood sugar regulation, appetite suppression, and fat loss. Additionally, researchers are exploring its possible cardiovascular advantages, which could enhance its value as a research tool.
Researchers interested in studying retatrutide for weight loss and other uses are encouraged to obtain the peptide in research-grade form from our most trusted supplier.

Disclaimer: Peptideinfo.net contains information about products that are intended for laboratory and research use only, unless otherwise explicitly stated. This information, including any referenced scientific or clinical research, is made available for educational purposes only. Peptideinfo.net makes every effort to ensure that any information it shares complies with national and international standards for clinical trial information and is committed to the timely disclosure of the design and results of all interventional clinical studies for innovative treatments publicly available or that may be made available. However, research is not considered conclusive. Peptideinfo.net makes no claims that any products referenced can cure, treat or prevent any conditions, including any conditions referenced on its website or in print materials.
To the extent that Peptideinfo.net references a product that is also a prescription medication, Peptideinfo.net does not does not offer medical diagnosis or treatment advice. The contents of Peptideinfo.net are intended exclusively for qualified researchers. Any individual seeking any advice on any prescription medication, or any disease or condition, is advised to refrain from using this site and consult their healthcare provider. Statements regarding products presented on Peptideinfo.net are the opinions of the individuals making them and are not necessarily the same as those of Peptideinfo.net.
Your access to Peptideinfo.net is subject to our terms of use.